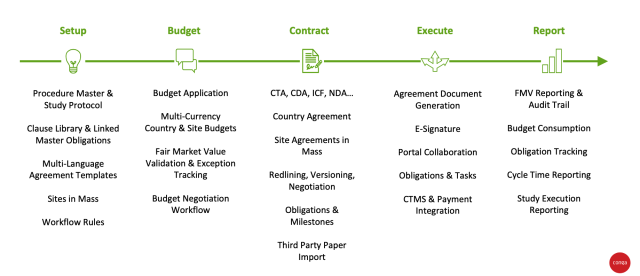
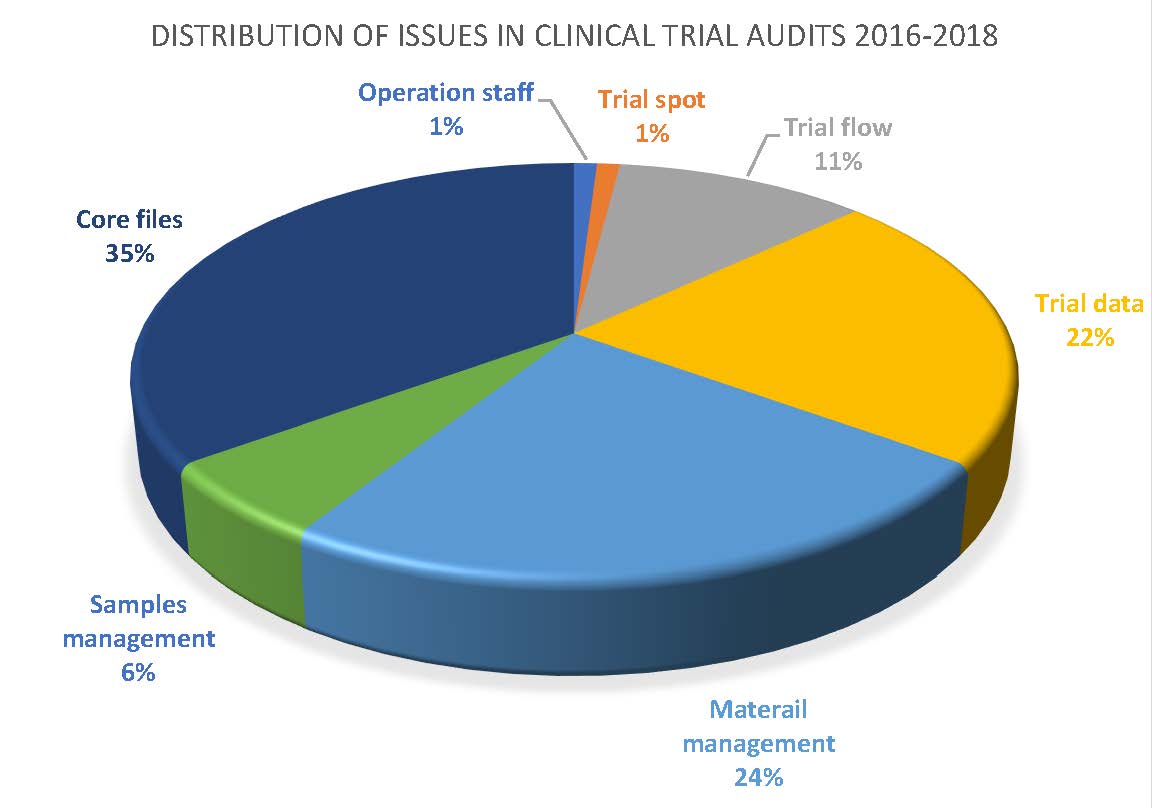
PDF) Project Management of Randomized Clinical Trials: A Narrative Review | Hamidreza Goodarzynejad - Academia.edu
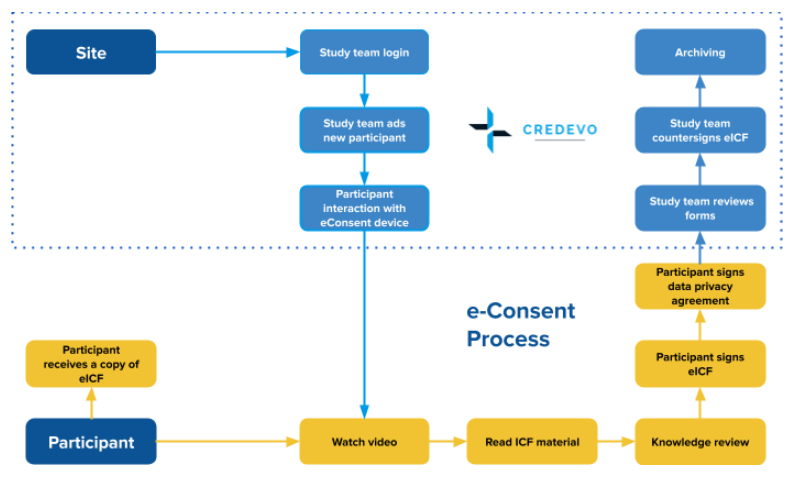
An enhanced participant information leaflet and multimedia intervention to improve the quality of informed consent to a randomised clinical trial enrolling people living with HIV and obesity: a protocol for a Study

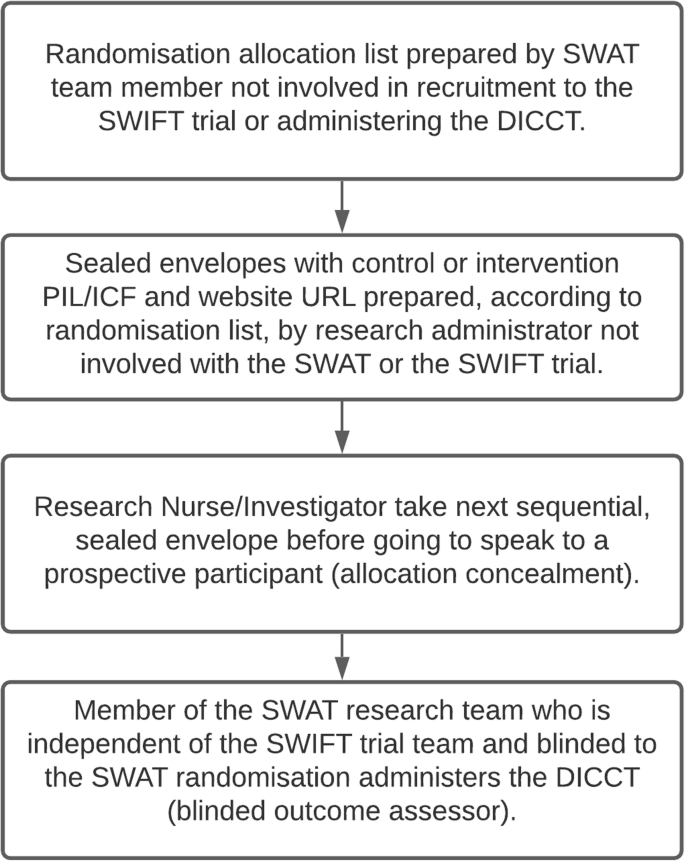
Considerations for obtaining informed consent. ICF, Informed consent... | Download Scientific Diagram

What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH