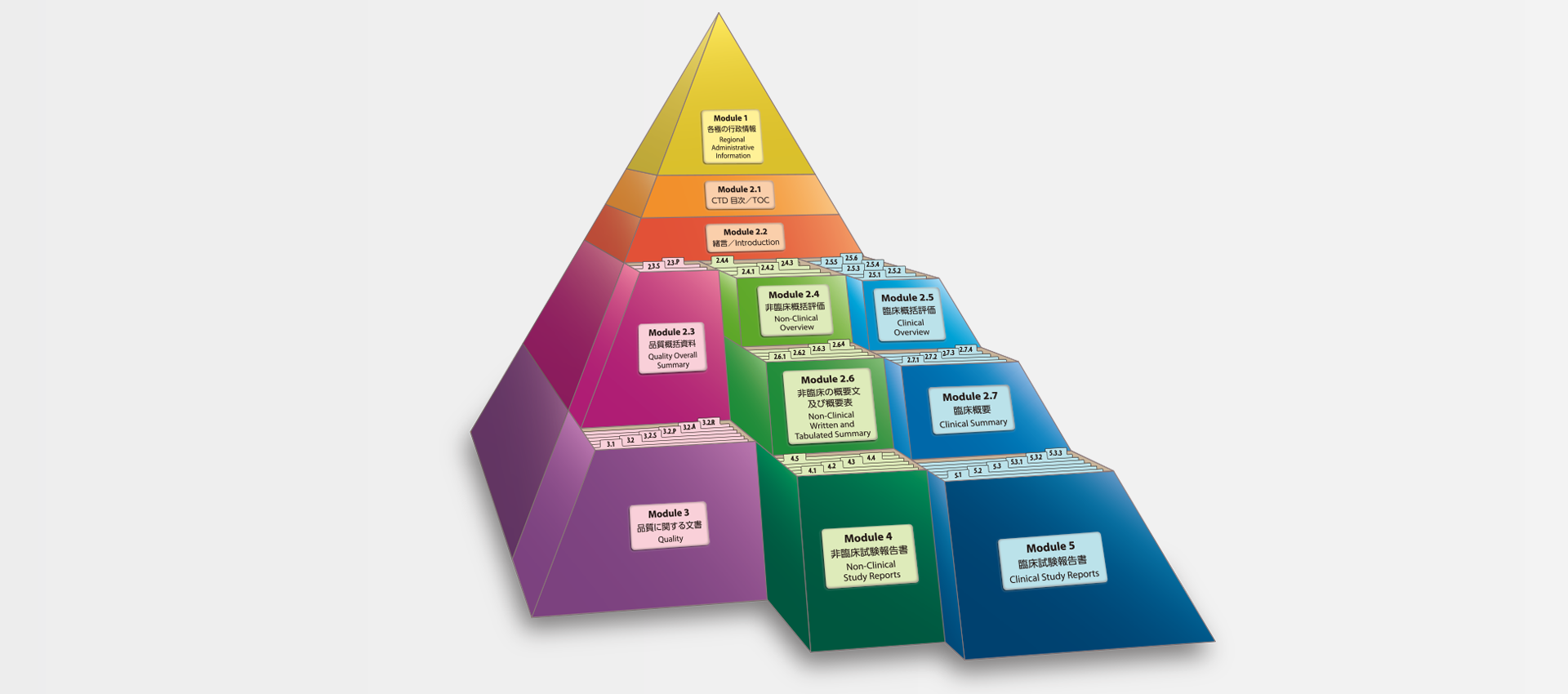
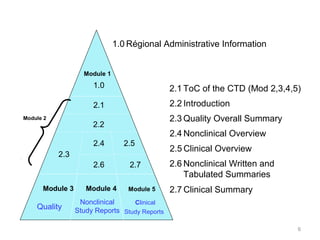
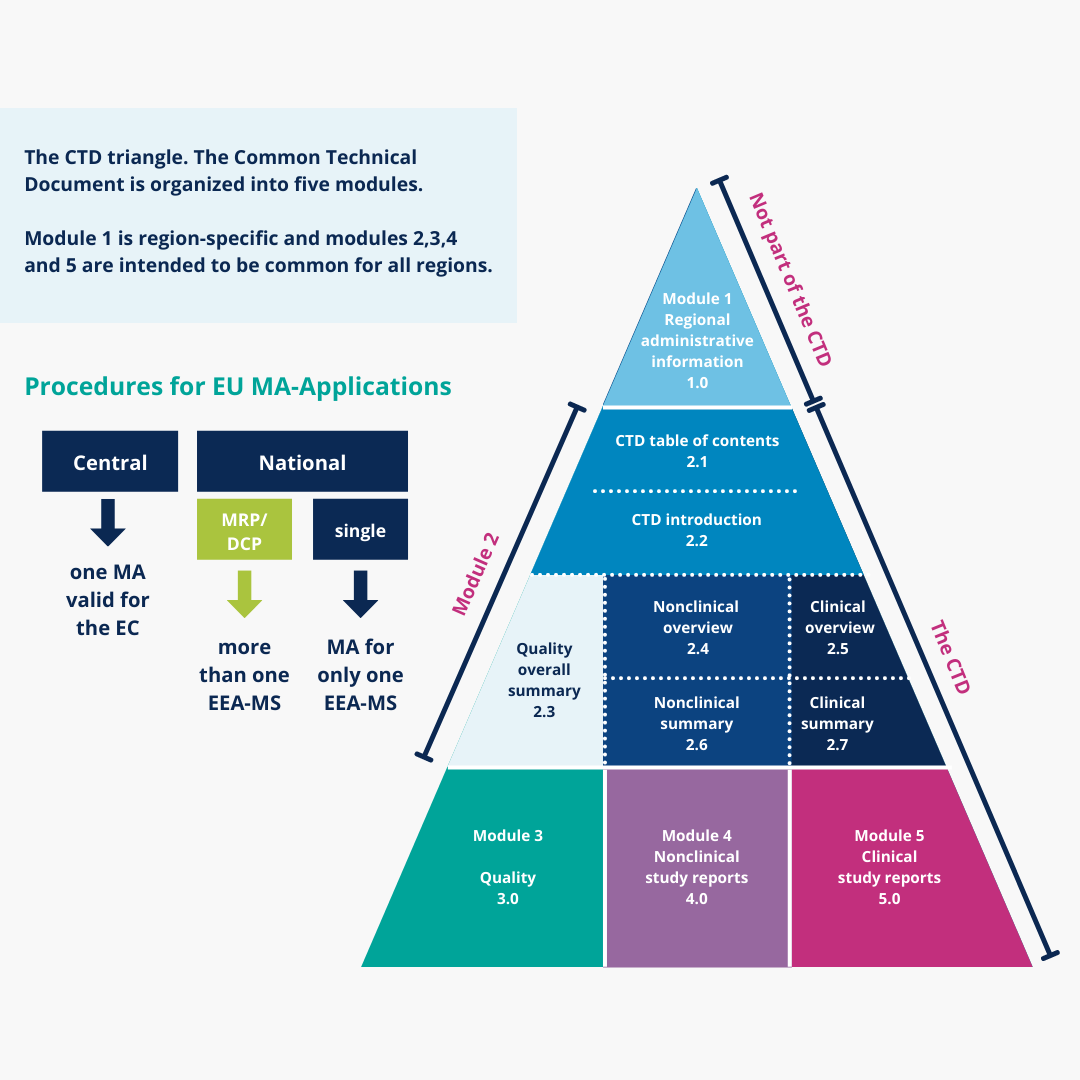
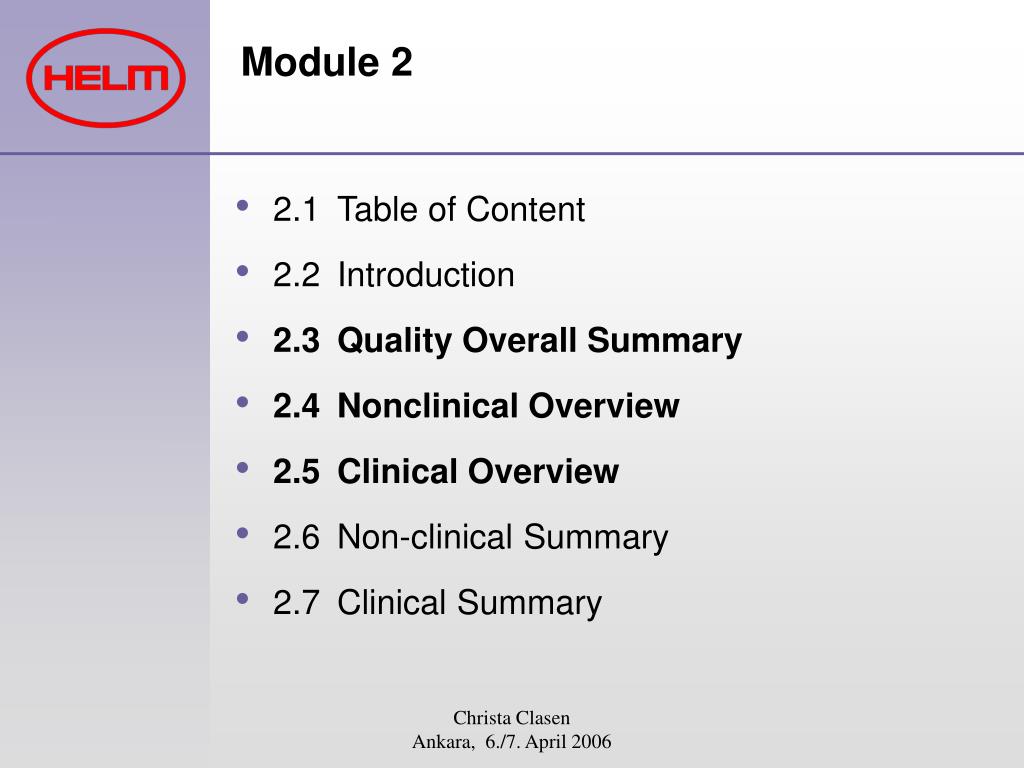
Clinical Study Reports Quality 2.7 Clinical Summaries 2.5 Clinical Overview 2.3 Quality Overall Summary 22.6 Non-Clinical Summar

TuraSkills shares tip for writing #Module 2.5 #Section 2.5.2 #Overview of Biopharmaceutics #Clinical overview #CTD overvie… | Writing tips, Marketing data, Writing

The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH

FFUL LisbonHilde Boone 29 May 2003 EMEA 1 Implementation of the CTD in Europe & EMEA Experiences Evaluation and Regulation of Medicines & Health Products. - ppt download
Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substan

Volume 2B Notice to Applicants. Medicinal products for human use. Presentation and format of the dossier. Common Technical Document (CTD) - PDF Free Download

The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH

Benefits vs. Risks: Telling the Story in the Clinical Overview May be Changing - IMPACT Pharmaceutical Services, Inc.