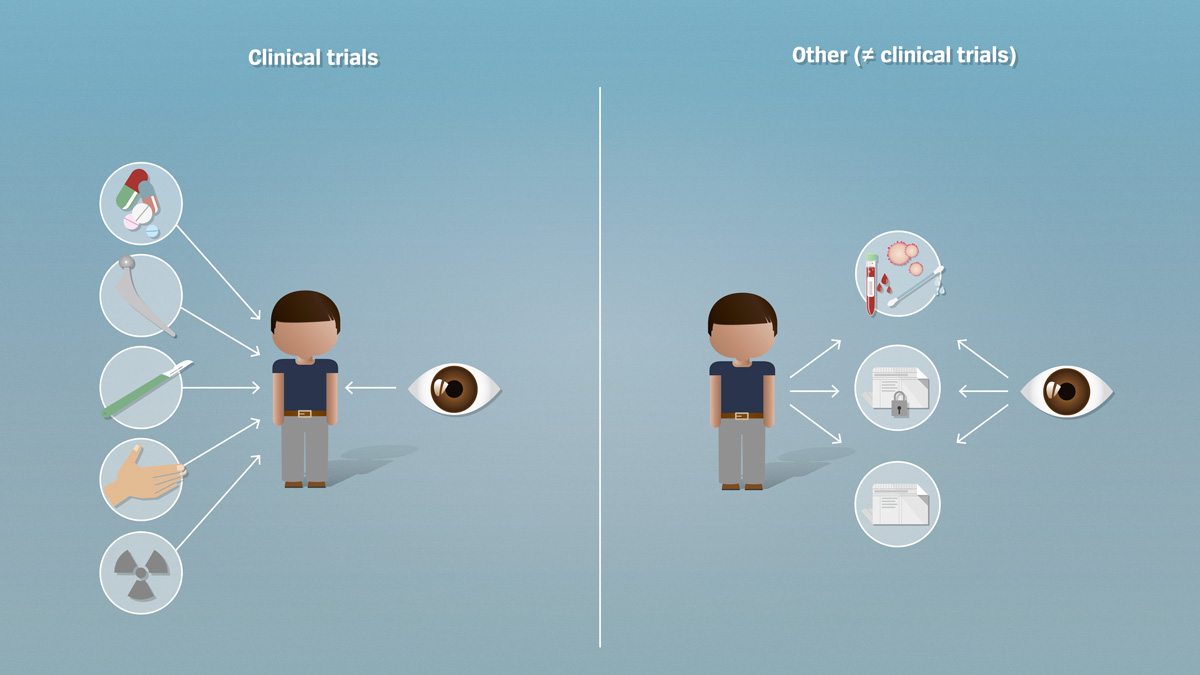
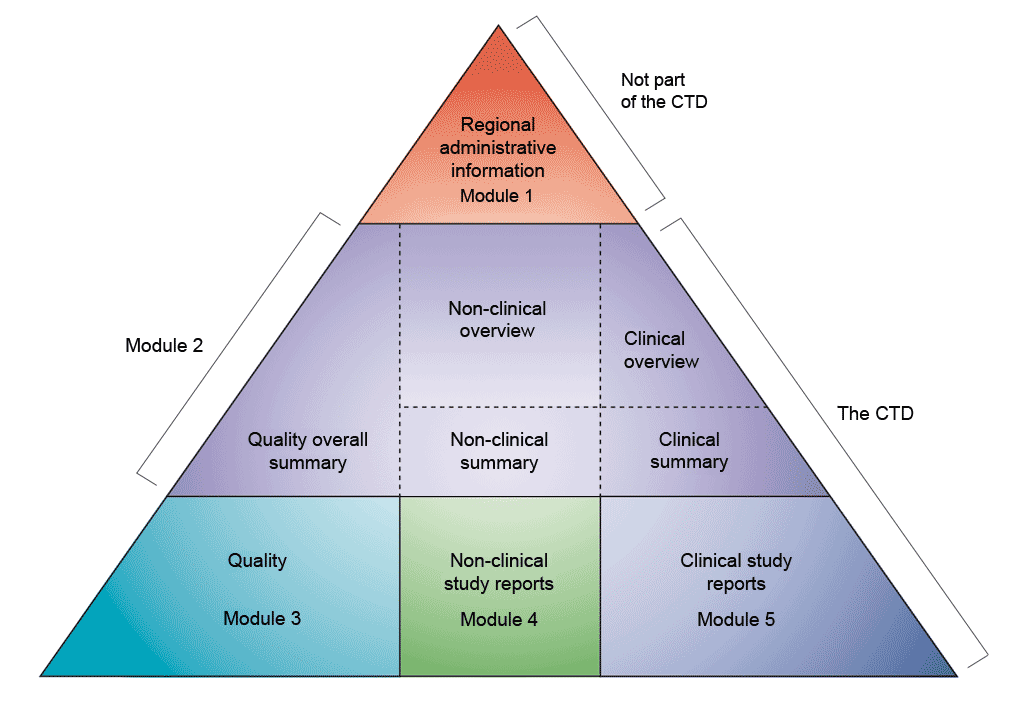
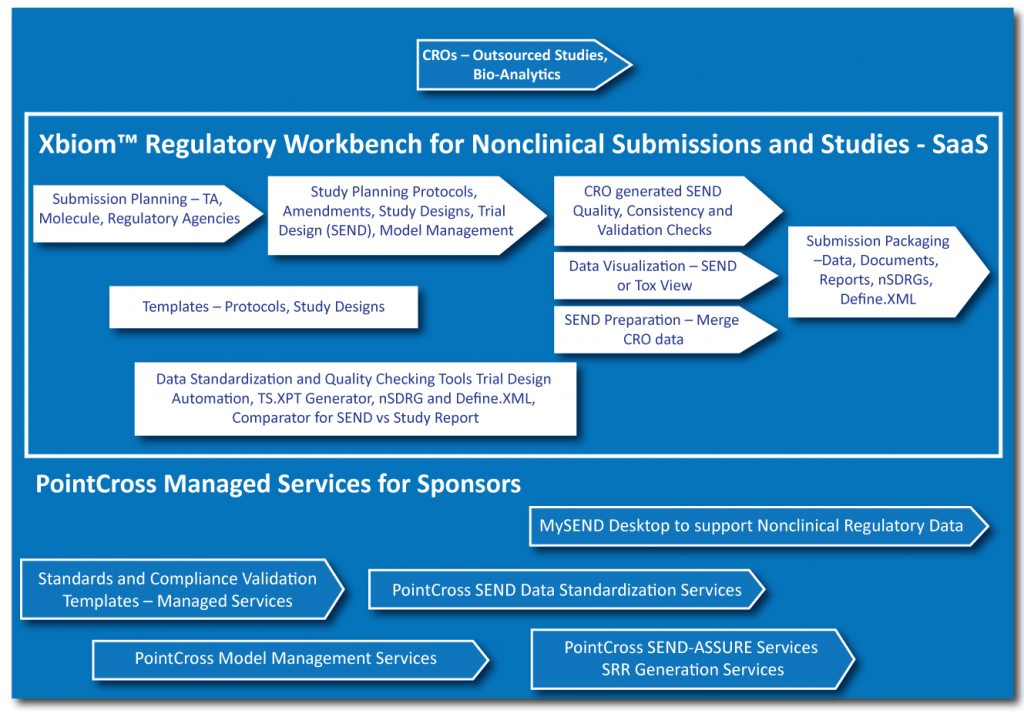
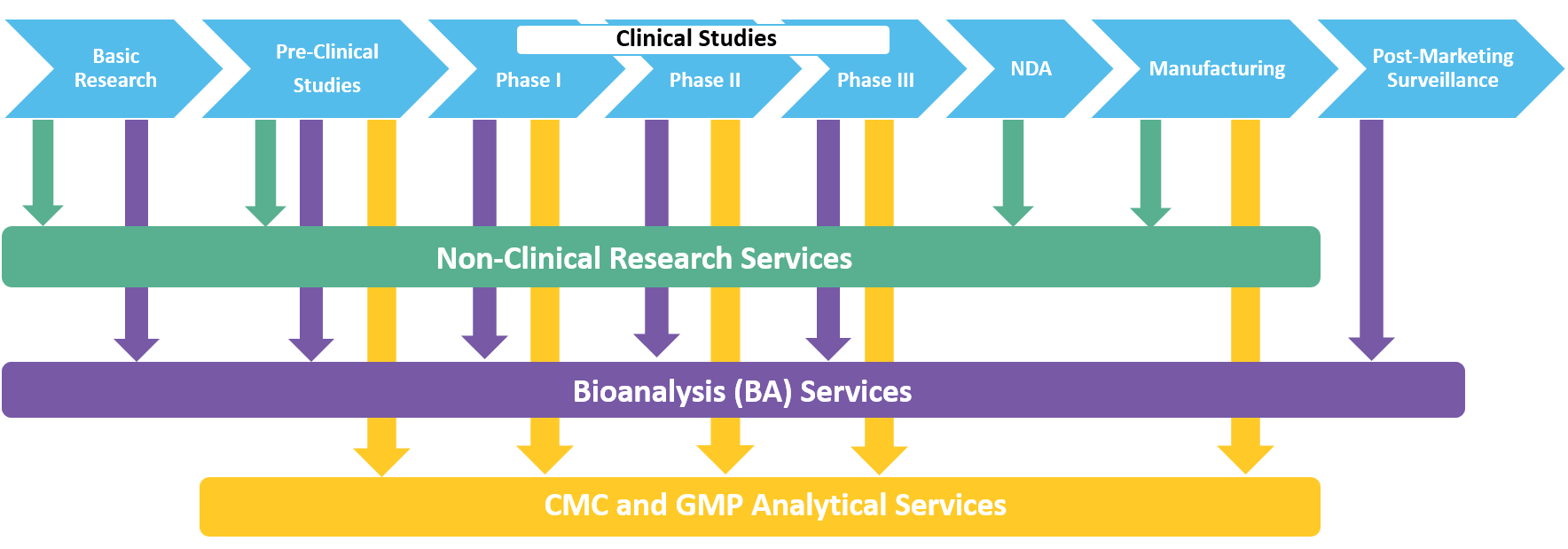
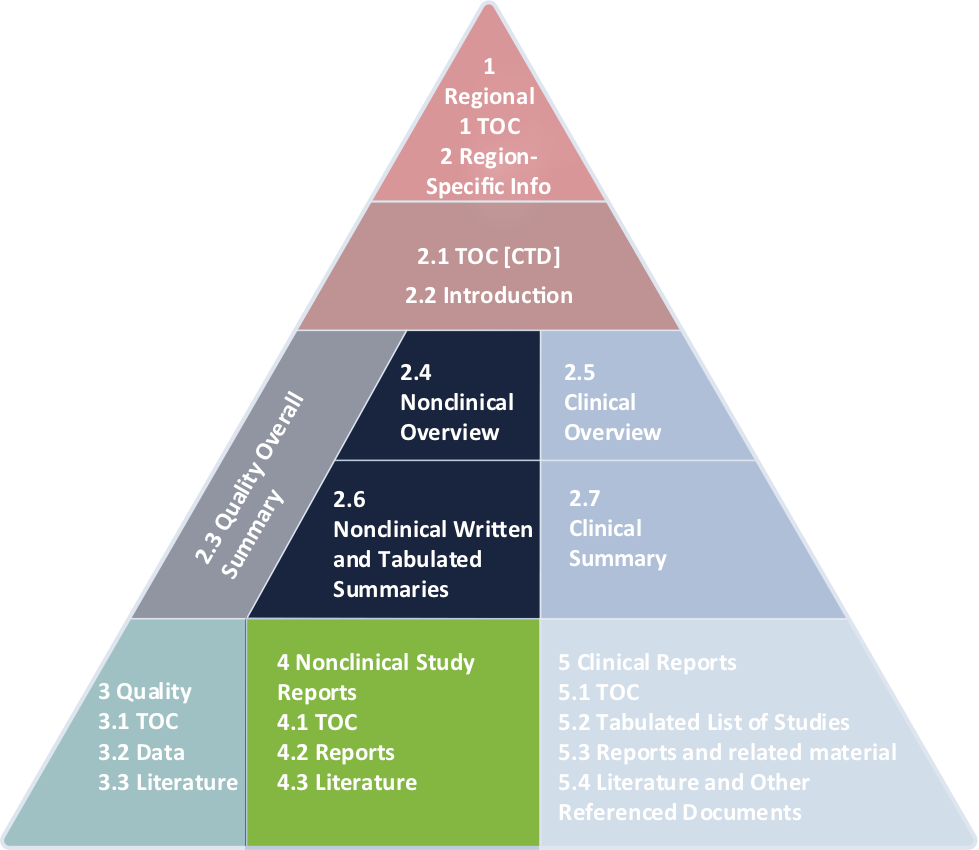
Regulatory aspects of prospective and retrospective clinical research in France in 2018 - ScienceDirect
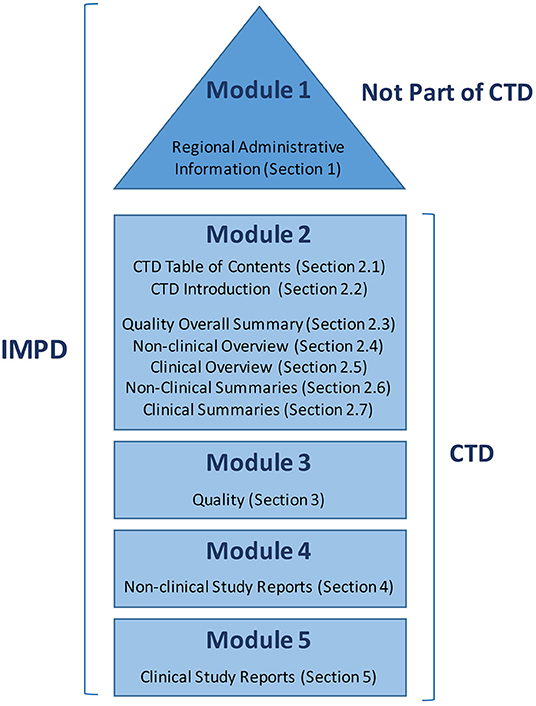
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience

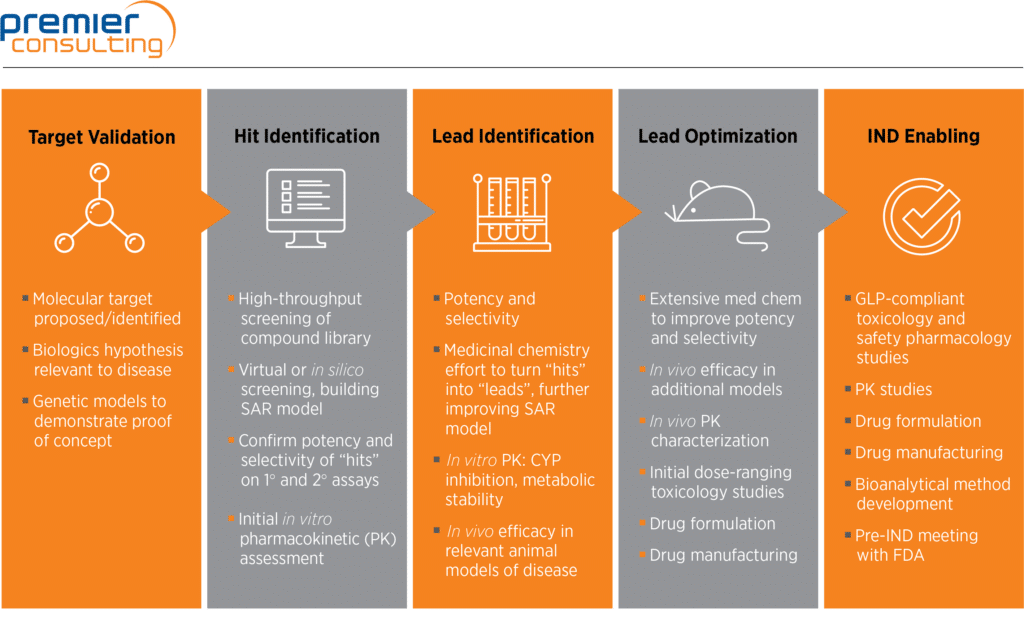
Steps of non-clinical studies in drug development process. GLP: Good... | Download Scientific Diagram
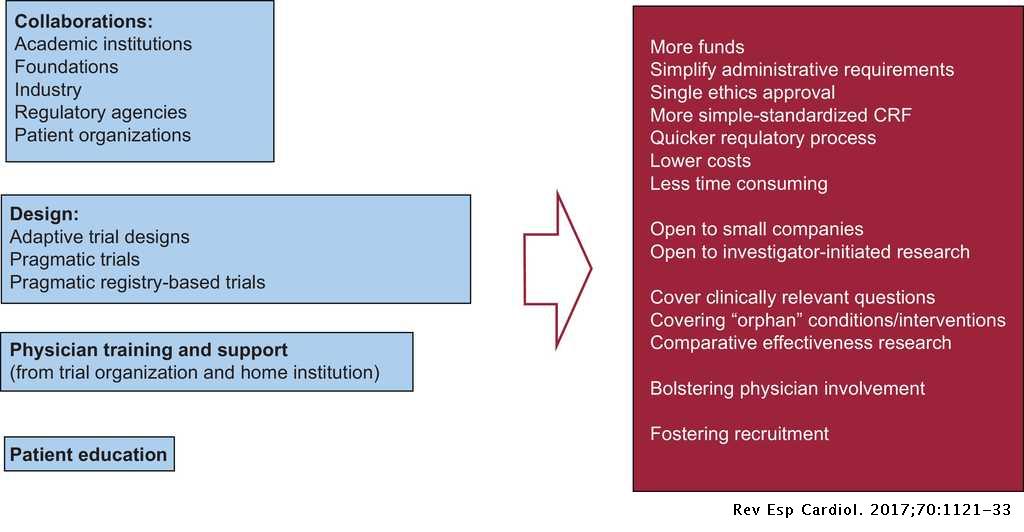
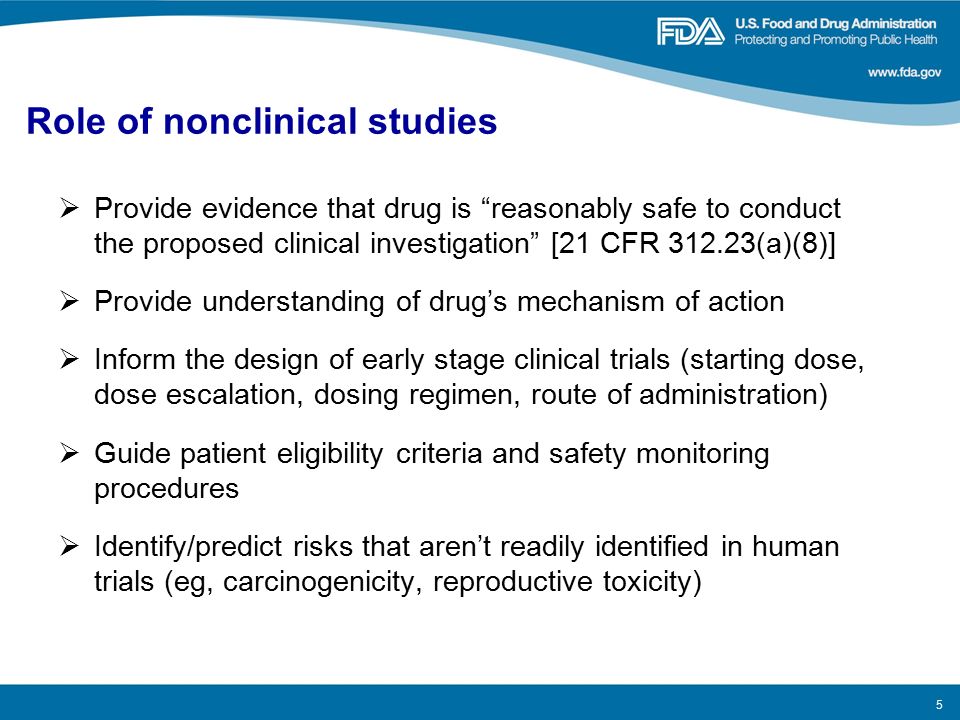
From Nonclinical Research to Clinical Trials and Patient-registries: Challenges and Opportunities in Biomedical Research | Revista Española de Cardiología

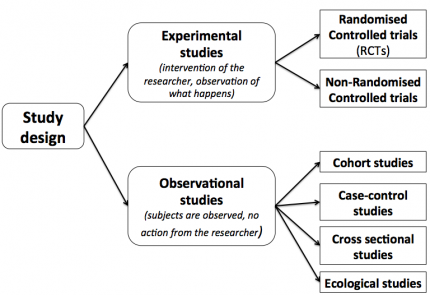
Statistical controversies in clinical research: limitations of open-label studies assessing antiangiogenic therapies with regard to evaluation of vascular adverse drug events—a meta-analysis - Annals of Oncology
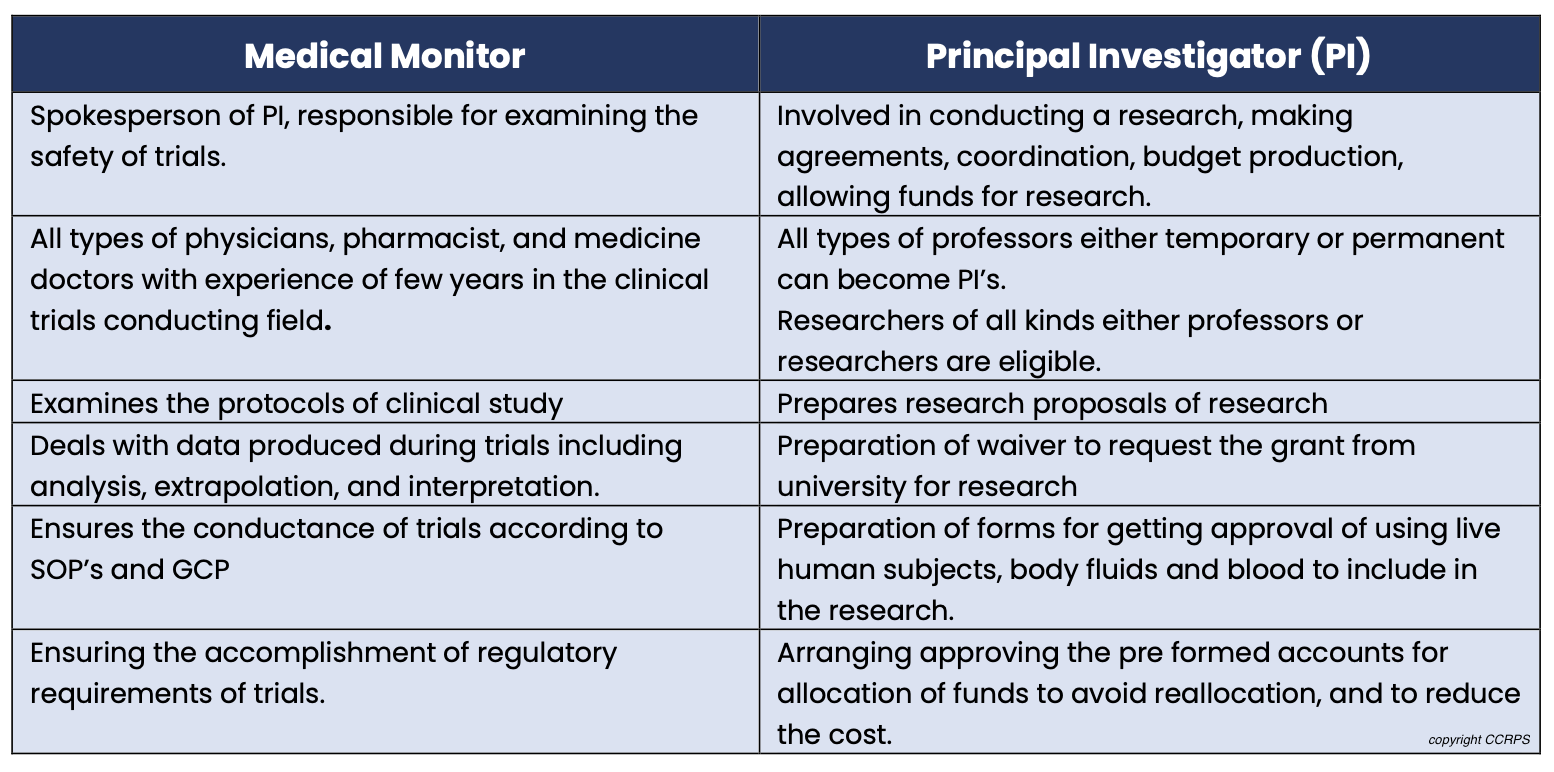
Medical Monitoring in Clinical Research - Non Clinical Physician Jobs — Clinical Research Certification

Regulatory compliance: How to shape a non-clinical development program and paediatric requirements - YouTube